The human race is attuned to experiencing the ordinary on a day-to-day basis, but the world, at times, has other plans. More notable days, such as November 16 of last year, break this monotony by introducing groundbreaking discoveries, debunking outdated claims, or overcoming world standards and records. On that day at Versailles, France, 60 countries formally proposed the changing the bases of measurement for four of the seven physical units of the Système International d’Unités, or the International System of Units (SI).
The four aforementioned units are the kilogram, ampere, mole, and kelvin, which serve as the base units of measurement for mass, electric current, amount of substance, and temperature, respectively.
The other SI units, candela, which measures luminous intensity or the brightness of the source of visible light; meter, the basis for length; and second, which is the basic unit of time, already use fundamental constants of nature. With the redefinition, all SI units can now be easily measured with precise instruments such as the Kibble Balance which measures Planck’s constant, instead of referring to physical phenomena for bases.
The change was enacted only last May 20, which was also celebrated as World Metrology Day, hence The LaSallian looks into its implications and effects on the fields of science and technology and explores the beginnings of the transformation.
From the grassroots
Way before the SI units came to be, the human body served as the basis for measurement. However, there was a common problem involved. Using the human body as basis meant that the standard of measurement was inconsistent; one person’s arm may be longer or shorter than another, or someone may hold much more grain. Because the units of measurement in ancient societies were not uniform, loopholes or discrepancies were not avoided.
With the rise of global trade and industrialization, especially in the 1800s and 1900s, there was a need to develop a more precise and standardized system of measurement, which everyone agreed with and which everyone can use.
Système International d’Unités, also known as the metric system, was born out of the French Revolution when revolutionaries abolished the preceding measuring system in place, and was adopted by the 11th General Conference on Weights and Measurements (CGPM, derived from Conférence Générale des Poids et Mesures) in 1960. Unlike the previous system, the SI units, that time, provided more concrete bases for measurement, such as full radiator brightness and thermodynamic temperatures. The SI units are widely used and adopted in all countries except in the United States, Canada, United Kingdom, Myanmar, and Liberia.
Relieving the interim
When the four previously mentioned units were updated in May, the interim definitions were not obsolete per se, but there was a need to take precision and appropriateness into consideration.
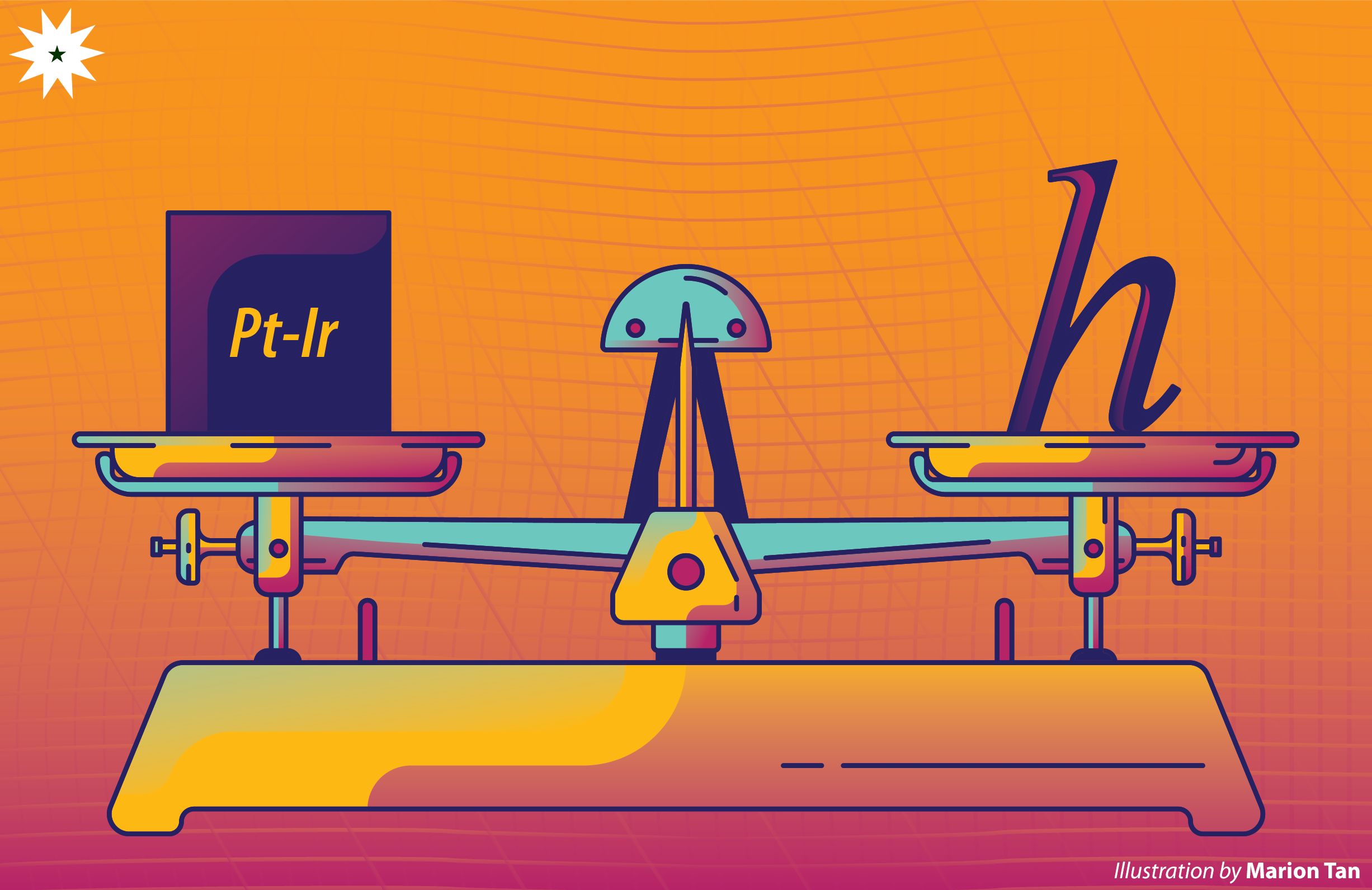
Dr. Lawrence Belo of DLSU’s Chemical Engineering Department discusses that before the redefinition, the last basis of the kilogram or the International Prototype of the Kilogram (IPK) was a platinum-iridium cylinder that was created to weigh exactly one kilogram and was made with materials that are resistant to corrosion to prevent any loss in mass. However, despite the best efforts in maintaining the IPK, it was discovered that its mass has decreased by around 50 micrograms since its creation in the 19th century.
The new basis for the kilogram is now Planck’s constant, usually denoted by the letter h, which has a value of 6.626176 × 1034 joule seconds. It is now being used as basis because of the units associated with it—joule second; joule is already a derived unit, which means it is made up of a specific combination of base SI units—meters, seconds, and kilograms. Belo emphasizes that while the change in the kilogram’s definition may be “small”, it is still “a change nonetheless and therefore not constant”.
Meanwhile, the ampere is redefined such that the elementary charge, which is the electrical charge carried by a single electron, is equal to 1.602176634 x 10-19 coulombs; the kelvin is set at the fixed numerical value of the Boltzmann constant, 1.380649 x 10-23 joule per kelvin; while Avogrado’s constant is equated to be 6.02214076 x 1023 per mole.
Moving forward
Belo mentions that professors such as himself now have to revise books and lecture materials to reflect the change, as these units will be the standard for all future measurements. In a larger scale, the change can now ensure heightened precision and accuracy even in creating the simplest technologies and in measuring the most basic substances.
Because of the new bases of the SI units, these references can, more or less, remain the same throughout the rest of human history, ensuring consistency.
